How India’s vaccine drive went horribly wrongon May 14, 2021 at 12:07 am
A botched-up plan for procuring jabs has dried up stocks and sent prices soaring on the private market.
image copyrightGetty Images
It took 31-year-old Sneha Marathe half a day to book an appointment online for a Covid vaccine.
“It was a game of ‘fastest finger first’,” she says. “The slots filled up in three seconds.” But the hospital cancelled her slot at the last minute: they had no vaccines. Ms Marathe went back to try for another appointment.
All 18-44 year-olds in India have to register on the government’s CoWin platform to get vaccinated. With demand for jabs far outstripping supply, tech-savvy Indians are even writing code to corner elusive appointments.
Ms Marathe can’t code, but she is among millions of Indians who are on the right side of the country’s digital divide – unlike hundreds of millions of others who don’t have access to smartphones or the internet, currently the only route to a jab.
Prime Minister Narendra Modi’s federal government has opened up vaccinations for some 960 million eligible Indians without having anything close to the required supply – more than 1.8 billion doses.
Worse, the severe shortage comes amid a deadly second Covid wave and warnings of an impending third wave.
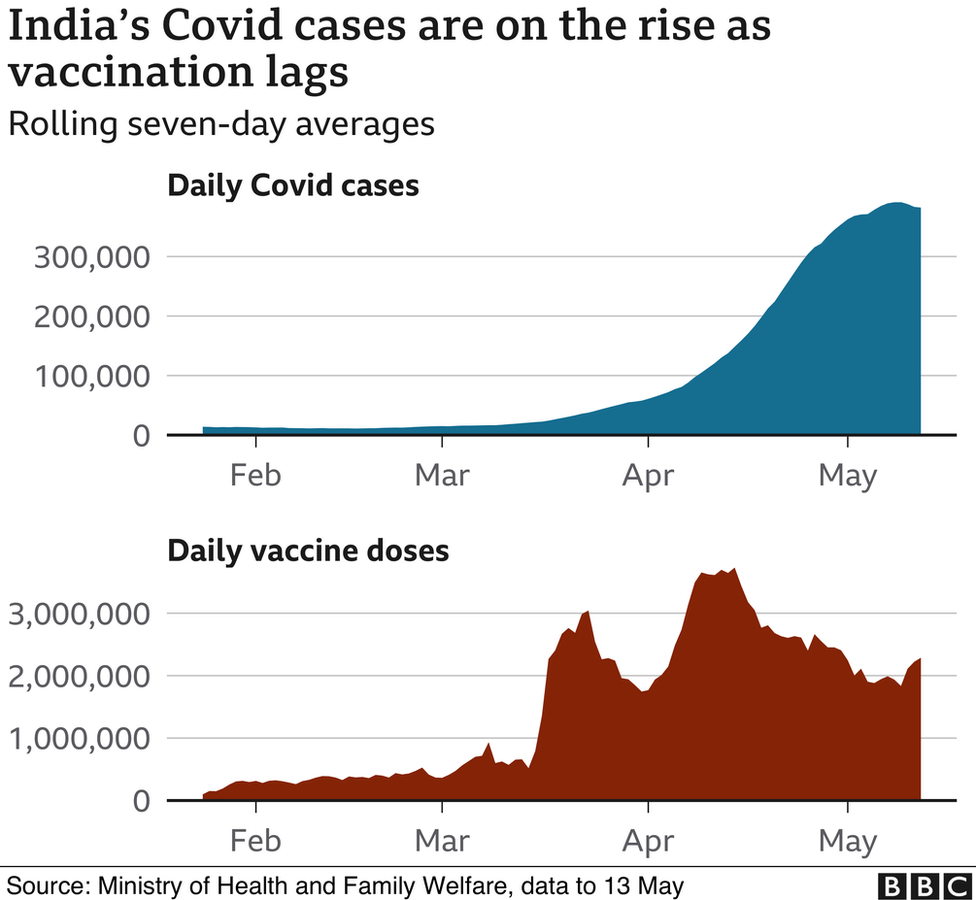
A cocktail of blunders – poor planning, piecemeal procuring and unregulated pricing – by Mr Modi’s government has turned India’s vaccine drive into a deeply unfair competition, public health experts told the BBC.
How did the world’s largest vaccine manufacturer, often dubbed “pharmacy of the world” for generic drugs, end up with such few vaccines for itself?
“India waited till January to place orders for its vaccines when it could have pre-ordered them much earlier. And it procured such paltry amounts,” says Achal Prabhala, a co-ordinator with AccessIBSA, which campaigns for access to medicines in India, Brazil and South Africa.
Between January and May 2021, India bought roughly 350 million doses of the two approved vaccines – the Oxford-AstraZeneca jab, manufactured as Covishield by the Serum Institute of India (SII), and Covaxin by Indian firm Bharat Biotech. At $2 per dose, they were among the cheapest in the world, but not nearly enough to inoculate even 20% of the country’s population.
Declaring that India had defeated Covid, Mr Modi even took to “vaccine diplomacy”, exporting more jabs than were administered in India by March.
Contrast that with the US or EU, who pre-ordered more doses than they required nearly a year before the vaccines became available for immunisation.

image copyrightGetty Images
“This guaranteed vaccine manufacturers a market, gave them certainty to forecast supply and sales, and ensured that some of these governments got large quantities as quickly as possible, once the vaccines were ready,” Mr Prabhala says.
Unlike the US and the UK, India also waited until 20 April – well into the second wave – to extend a $610m financing line to SII and Bharat Biotech to boost production.
Another failure, according to Malini Aisola, co-convener of the All India Drug Action Network, was the decision not to enlist the vast swathe of India’s manufacturing capabilities – biologics factories, for instance, that could have been repurposed into vaccine production lines.
Again, four firms, including three government-owned ones, have only recently been given rights to make Covaxin, which is partially publicly-funded.
On the other hand, by early April, Russian developers of Sputnik V, had inked manufacturing deals with a host of Indian pharma companies, which are set to produce the vaccine.
As the sole buyer initially, the federal government could have held far greater leverage over pricing, Ms Aisola says.
“Centralised bulk procurement would have allowed the price to come down from $2. Instead it has gone up,” she adds.
This is because since 1 May, it has been up to individual states and private hospitals to broker their own deals with manufacturers.
Opposition parties have called it a “scam”, saying the federal government had abdicated its responsibility, opening up “debilitating competition among states”.
States have to pay double – $4 – the federal government’s rate for a dose of Covishield and four times as much for Covaxin – $8. This was after the two companies lowered prices for states as a “philanthropic gesture”. States are also competing for scarce stocks alongside private hospitals, which can pass on the costs to customers.
The result: a veritable free market for vaccines that have been developed and manufactured with both public and private funding. At private hospitals, a single dose can now cost up to 1,500 rupees ($20; £14).

Several states have now announced plans to import other vaccines from Pfizer, Moderna and Johnson & Johnson. But no manufacturer is able to guarantee supply in the next few months since richer countries have pre-ordered stocks.
Sputnik V has been approved, but it’s still unclear when the vaccines will be rolled out.
Some have accused SII and Bharat Biotech of “profiteering” during a pandemic, especially after receiving public funding.
But others say they took substantial risks and that the fault lies with the government. India is the only country where the federal government is not the sole buyer, and one of the few where vaccinations are not free.
But public health experts agree that SII and Bharat Biotech need to be more transparent about their manufacturing costs and their commercial contracts.
Ms Aisola says SII needs to disclose how it spent the $300m it received from the international Covax scheme and the Gates Foundation, funding which was meant to finance vaccines for low-income countries. SII has failed to do so, partly because India banned exports. The company is also fielding a legal notice from AstraZeneca for defaulting on its promise to send 50% of its supply to low-income countries.
Public health experts are also calling for scrutiny of the Indian government’s contract with Bharat Biotech, especially since the Indian Council of Medical Research has said it “shares” intellectual property (IP) for Covaxin, which it developed along with the company. But the jab costs more – often double – than Covishield.
“They say they share IP but what sort of an agreement did they sign? Does it give them [the government] the right to override any clauses in case of an emergency?” asks Dr Anant Bhan, a public health expert.
While India has supported waiving the patents on foreign-made vaccines, it has made no move to suspend it for Covaxin.
Contrary to its international position, it has opposed suggestions from opposition leaders to invoke compulsory licensing and allow other pharma companies to manufacture the approved vaccines, saying these measures would prove “counterproductive”.
Dr Bhan agrees that at this stage it would take time to transfer technology and build capacity in other pharma companies – but he also says it’s unclear why none of this was attempted earlier.
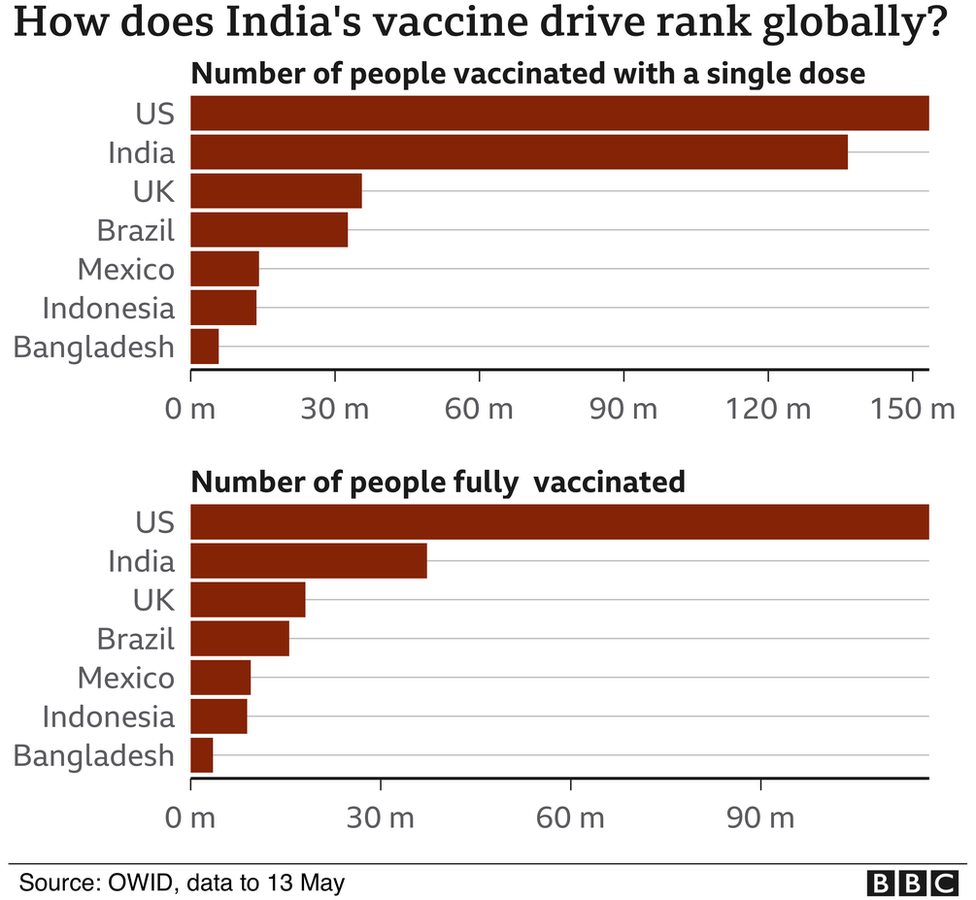
Vaccinating even 70% of India’s 1.4 billion people was always going to be a long exercise in planning and patience. But given the country’s strong record on immunisation, it was not an impossible task, Dr Bhan says.
However, why the government chose to rely on just two companies who can now control supply and dictate prices is a question that few have answers to.
Charts by Shadab Nazmi


